Supramolecular Chemistry and Ion Mobility Mass Spectrometry
- goodgreenlife

- Jan 7
- 5 min read
What is supramolecular chemistry?
Supramolecular chemistry isn’t sharply defined as organic chemistry or physical chemistry. It is defined more by how molecules interact rather than what they are made of. A supermolecule is composed of molecules and/or ions held together by weak non-bonding interactions. Though reversible and weak these interactions are numerous and may dramatically change the properties of constituent parts of the association.
To give an understanding of the non-bonding interactions, figure 1 lists relative strengths of forces.
Intermolecular Force | Strength (kJ/mol) | Notes |
Hydrogen bonding | 4 - 50 |
|
Ion-dipole forces | 2 – 8 | Environmentally dependent |
Pi-Pi | 40 – 99 | Phenylalanine, Tyrosine |
Van der Waals | <1 – 15 | Short range, additive |
Cation-cation bonding | 100 – 600 | Rough estimate |
Salt bridge | 5 – 6 |
|
Covalent bond | 200 – 400 |
|
Ionic bond | 1100 - 20000 | Lattice energies |
Fig1. Relative strength of forces. Note different sources give different relative strength due to different ways of calculating it, however the magnitude tends to be in the same ballpark.
Defining and measuring the intermolecular forces
Intermolecular forces observed between atoms and molecules can be described phenomenologically as occurring between permanent and instantaneous dipoles. Quantum mechanics is a fundamental unifying theory that is able to explain various types of interactions such as hydrogen bonding and van der Waals force. The Rayleigh-Schrodinger perturbation theory has been effective at this. Perturbation theory comprises methods for finding an approximate solution to a problem starting from the exact solution of a related simpler problem. Other ways of explaining and visualising this is the non-covalent interaction index (NCI).
NCI is a visualisation index based in the electron density (p) and the reduced density gradient (s). It is based on the empirical observation that non-covalent interactions can be associated with the regions of small reduced density gradient at low electronic densities. This approach allows hydrogen bonds, pi-pi interactions and van der Waals contacts to be visualised directly in real space, offering intuitive insight into weak interactions that are otherwise difficult to define quantitatively.
The strength of intermolecular forces is highly context dependent. Interactions are weakened in high dielectric environments such as water but strengthened in non-polar solvents or buried protein interiors. Dielectric constant measures a materials ability to store electrical energy in an electric field. A solvent’s dielectric constant indicates its polarity and ability to separate charges, with higher values meaning greater polarity and better stabilisation of ion making it good for polar compounds. For numerous weak non-covalent interactions a non-polar solvent is ideal.
In 1987 the Nobel Prize in Chemistry was awarded jointly to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen “for their development and use of molecules with structure-specific interactions of high selectivity”. As supramolecular chemistry spans very different size scales and method, it can be divided into subfields. To think about it from first principles, size dictates the physics it follows whether it be quantum mechanics or more classical Newtonian physics. This dictates the experimental tools and so different supramolecular objects require different approaches. According to Helena Dodzioks book the field could potentially be split into three areas differing by the objects studied and experimental techniques used. Note this book was written in 2000, a great many things may have been discovered in the past 3 decades.
Inclusion or Host-guest chemistry
The first area defined as inclusion complexes deal with small aggregates. To give examples this may be a crown ether + metal ion, a host molecule with a drug molecule. So, one host, and one or more guests with 1:1 or 1:2, etc stoichiometry.
Crystal Engineering
This is supramolecular chemistry in the solid state. Instead of one molecule to a crystal it is multiple components forming an engineered crystal lattice. Examples of this are Co-crystals or metal-organic frameworks. As crystal engineering uses supramolecular interactions to control materials, not just binding. To analyse these crystals, you would typically use XPRD or other x-ray crystallography techniques which provide atomic level structural information across extended lattices.
Aggregate chemistry
Between small host guest complexes and macroscopic crystals lies aggregate chemistry, which deals with mesoscopic systems. These assemblies are too big to be single molecules yet too small to be bulk crystals. Examples being mono layers, biolayers, micelles, and fibres. A defining feature of these systems is that the number of subunits is not fixed. Unlike host guest complexes, aggregates exist as distributions of sizes and shapes, making them analogous to polymers rather than molecules. Their behaviour is often governed by collective effects and emergent properties rather than single interactions.
What can you make?
As supramolecular systems are made from building black that are linked together by noncovalent interactions they can show stimuli-responsive behaviour. Changes in pH, temperature, light or chemical composition can trigger assembly, disassembly or functional switching. Chemical architecture like rotaxanes, catenanes and knots which are difficult to prepare can easily be prepared through templated synthesis. Many of these structures are difficult to prepare using purely covalent synthesis, yet can be made through templated supramolecular assembly. The discipline has only really been around for 50 years however it covers many areas of study.
Supramolecular chemistry now underpins a wide range of research areas, including:
Molecular machines and motors
Chemical sensing and recognition
Gas adsorption and separation
Nanoreactors and catalysis
Drug delivery and controlled release
In some cases, supramolecular assemblies can even catalyse their own formation. In autocatalytic systems, this leads to self-replication, crudely mimicking biological reproduction, as discussed in work by Luisi and co-workers (link).
How do you know you have what you think you have?
Depending on which class of molecular you have in the supramolecular field different tools are used for analysis. For molecules due to discrete energy levels more spectroscopic tools can be used to exploit quantum mechanics like IR, UV-Vis, NMR.
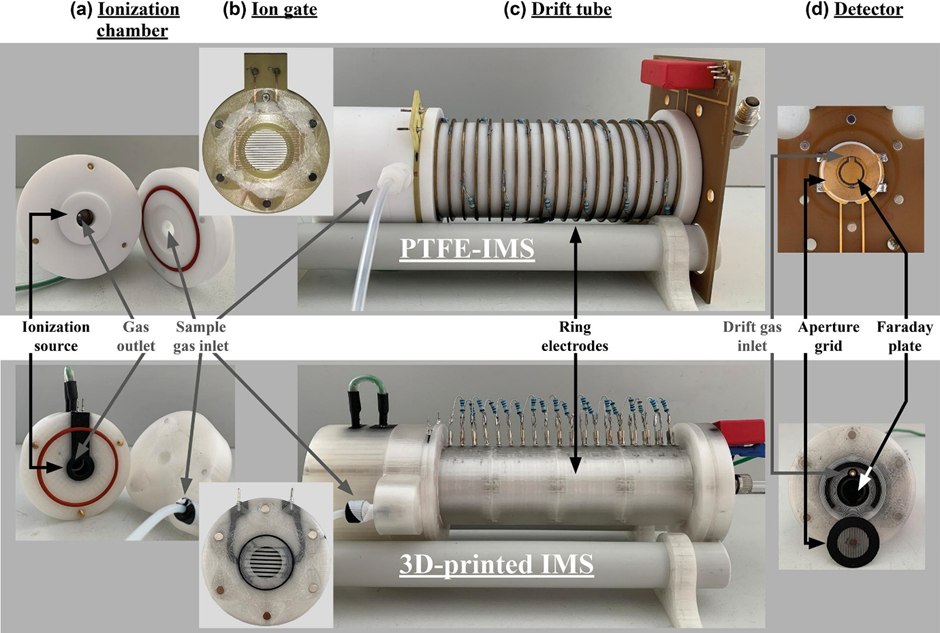
But for aggregates as these are larger molecules and ensembles with no singular molecular formula other tools are required. Methods like ion mobility mass spectrometry (IM-MS), or Xray diffraction are required.
IM-MS is particularly powerful because it provides simultaneous information on mass, chape and population making it well suited to characterising supramolecular systems. It also excels at analysing complex mixtures without prior isolation. By separating ions according to both m/z ratio and CCS (Collision Cross Section), IM-MS can resolve overlapping species, conformers and assembly states that would otherwise be indistinguishable. This makes it especially valuable for studying dynamic supramolecular systems and reaction networks.
How do you apply machine learning to this?
There’s a lot to this topic and in the Rijs group different algorithms have been applied to help elucidate structures. CREST-CENSO (Conformer-rotamer Ensemble Sampling Tool – Command line Energetic Sorting) algorithms have been applied to elucidate the structures of metalated Glyphosate Dimers. These are powerful, multi-level computational chemistry approach used for the automated exploration of molecular conformation space and the subsequent energetic refinement of the resulting structures. They also use threshold density functional theory which balances accuracy and computational cost.
What are the frontiers of supramolecular chemistry?
Supramolecular chemistry is rapidly developing and is shifting away from static structures towards systems-level behaviour. Researchers are focusing on topics like: how does the system respond to perturbation, can it adapt, evolve, or store information, can complexity itself be functional.

By combining supramolecular design with techniques such as IM-MS and machine learning, chemists are beginning to treat chemical mixtures as information-processing systems, opening a new chapter in systems chemistry that blurs the boundary between chemistry, biology, and data science.



Comments